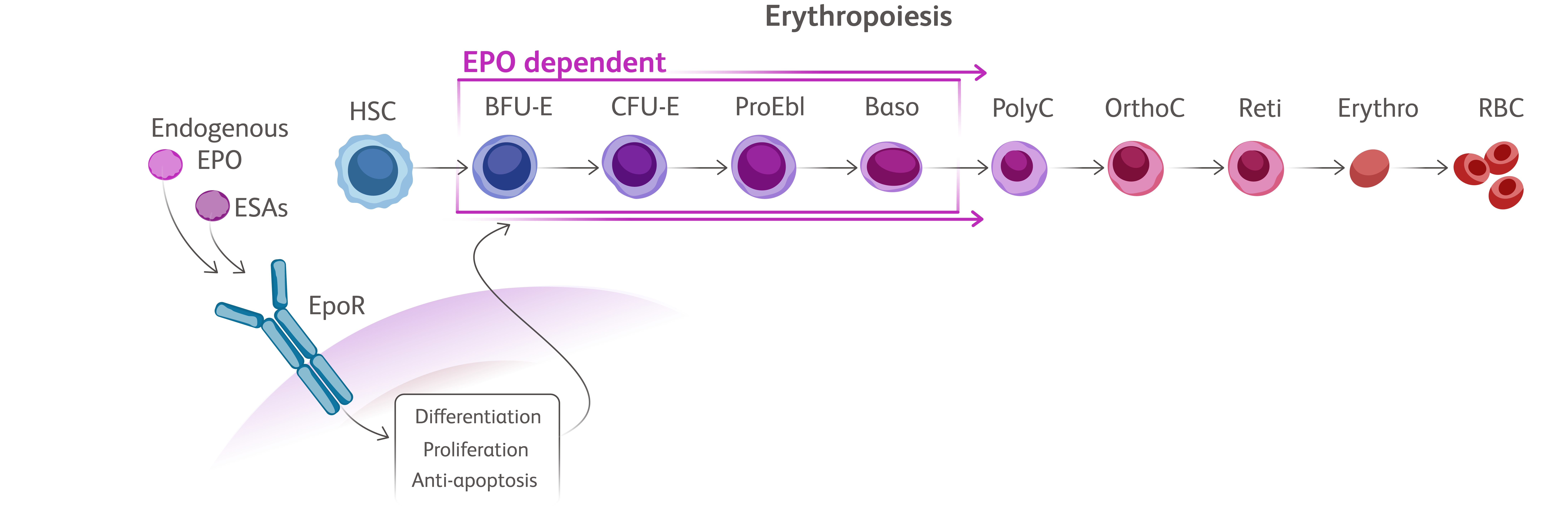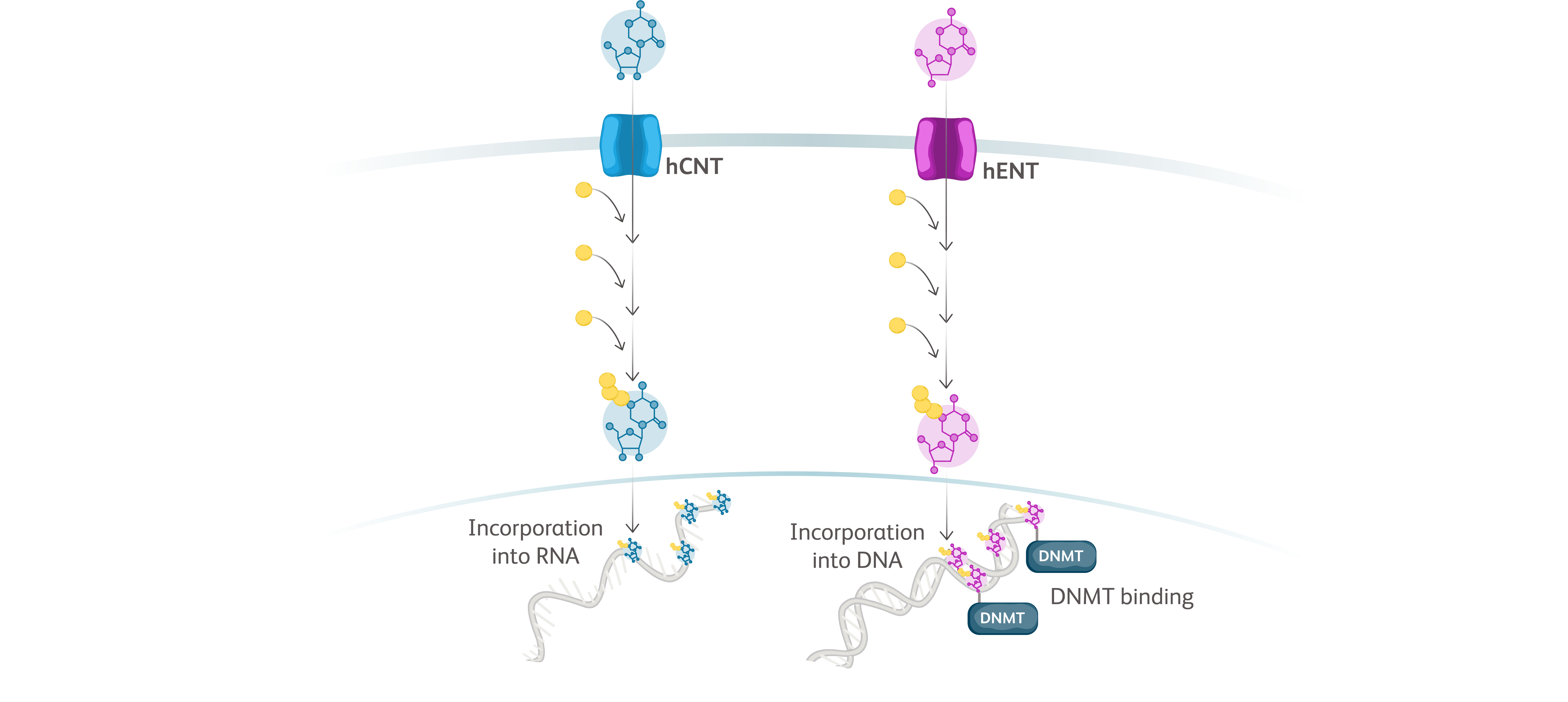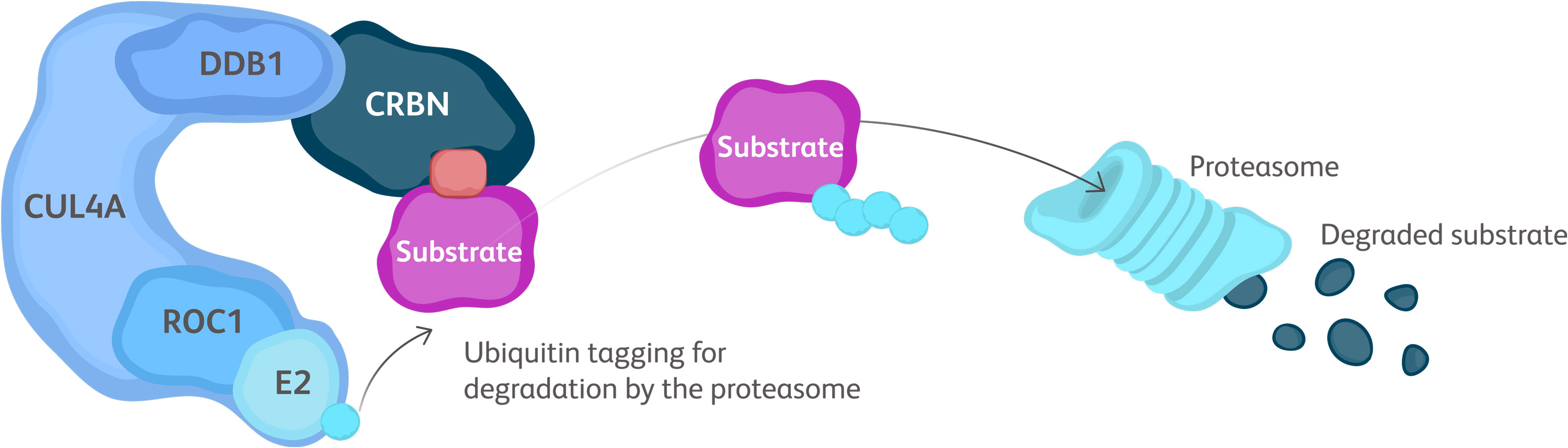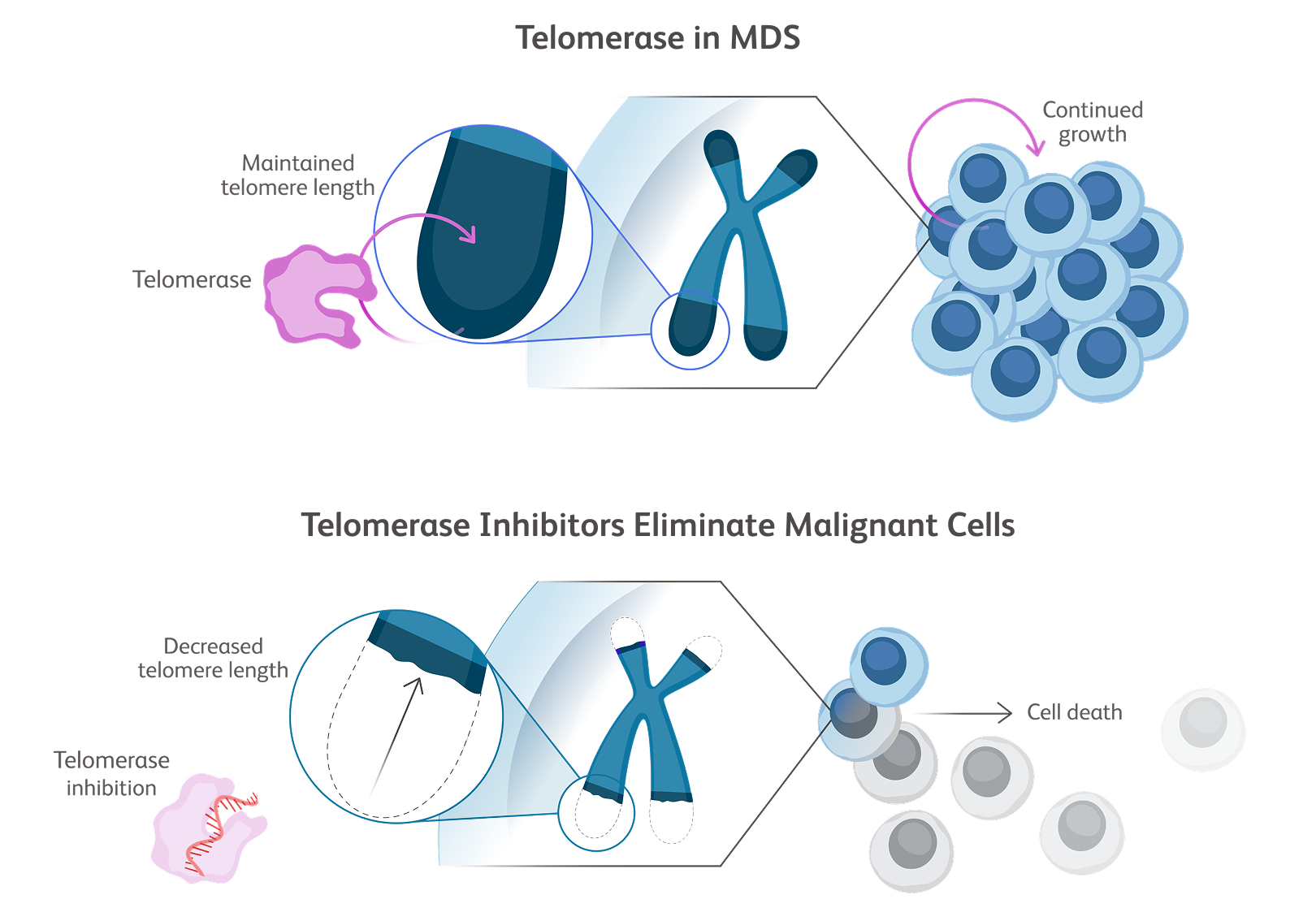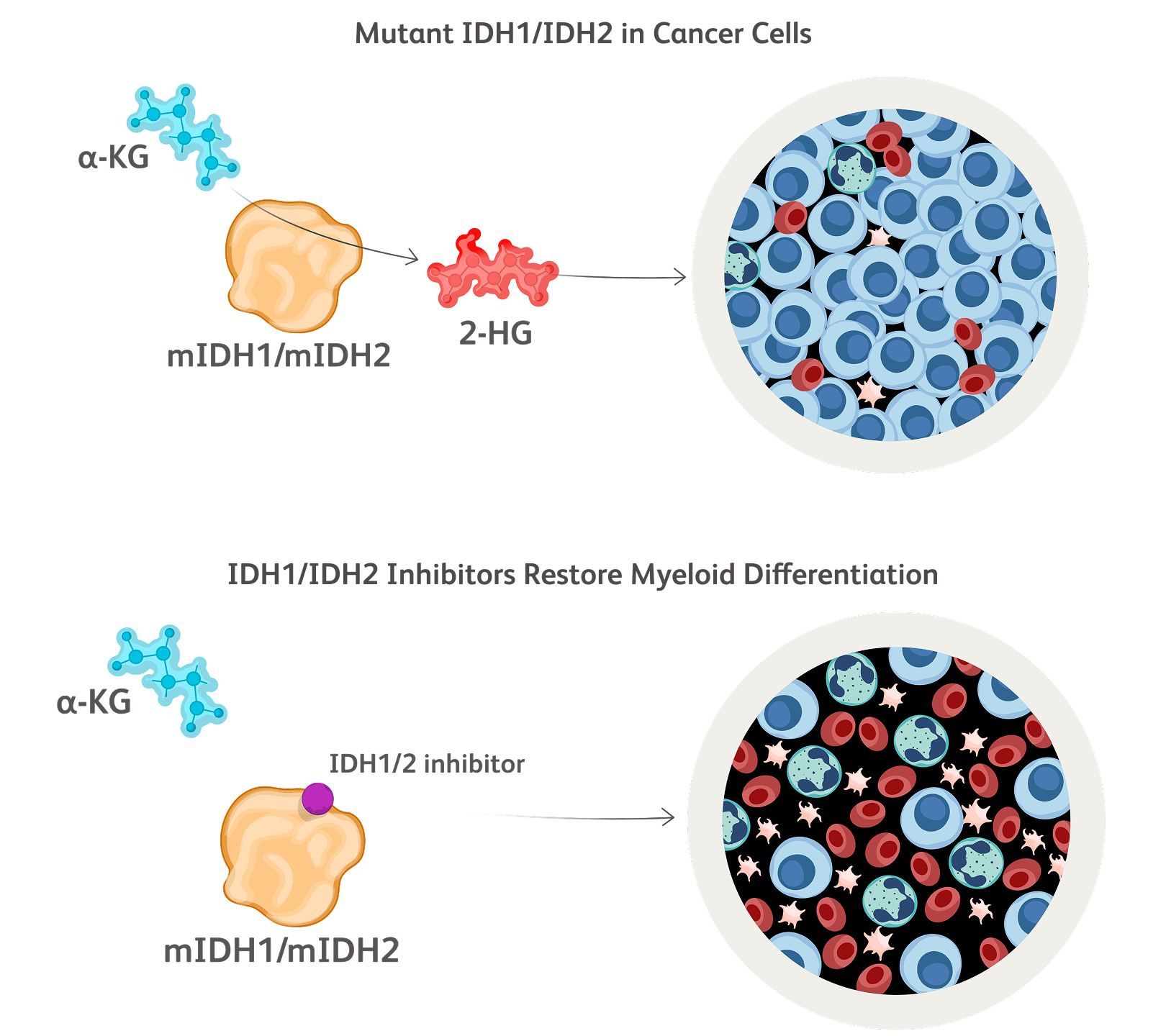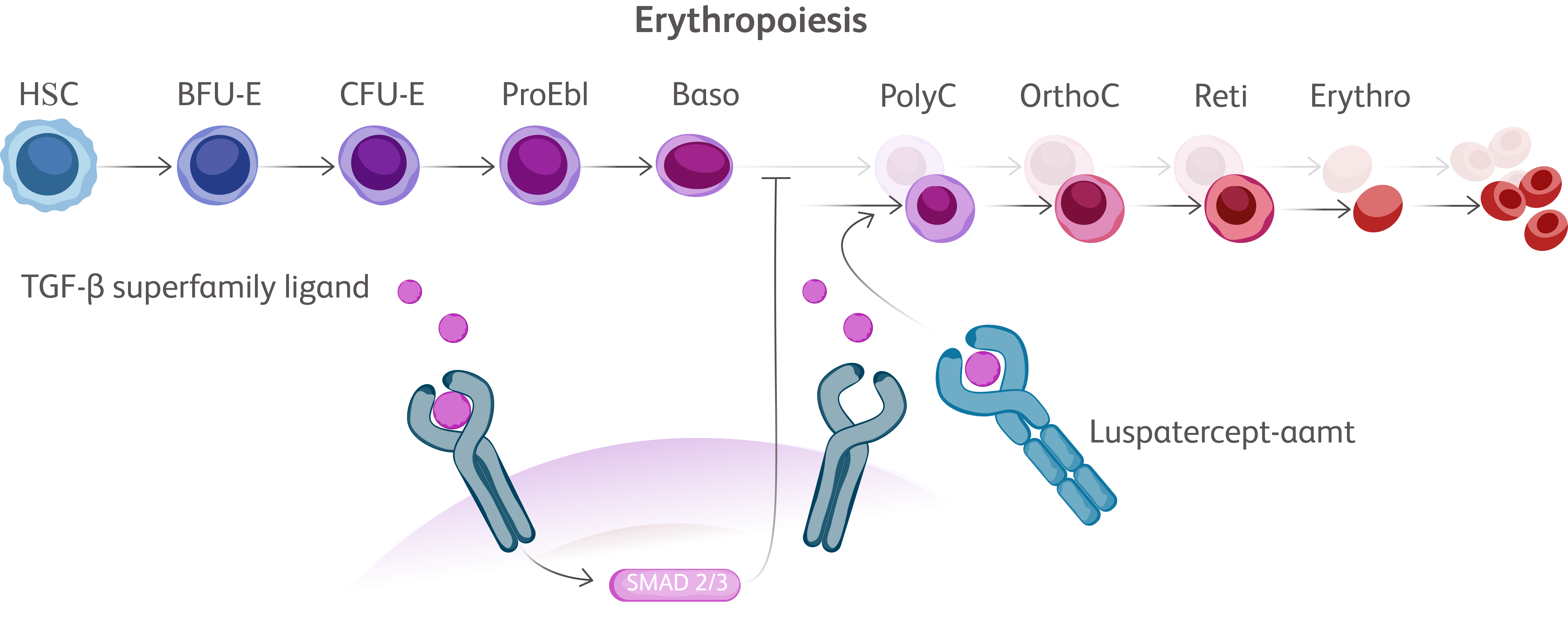
Baso, basophilic erythroblast; BFU-E, burst-forming unit erythroid cells; CFU-E, colony-forming unit erythroid cells; Erythro, erythrocyte; HSC, hematopoietic stem cell; OrthoC, orthochromatic erythroblast; PolyC, polychromatic erythroblast; ProEbl, proerythroblast; Reti, reticulocyte; TGF, transforming growth factor.
EMAs have been designed to bind TGF-β superfamily ligands leading to reduction in SMAD signaling allowing for erythroid maturation through differentiation and increased erythroid precursors, thereby increasing red blood cell (RBC) production.Kubasch AS, et al. Blood Adv. 2021;5(5):1565-1575. Vinchi F, Platzbecker U. Hemasphere. 2024;8(3):e41.